◆ 'Chainmail' catalyst
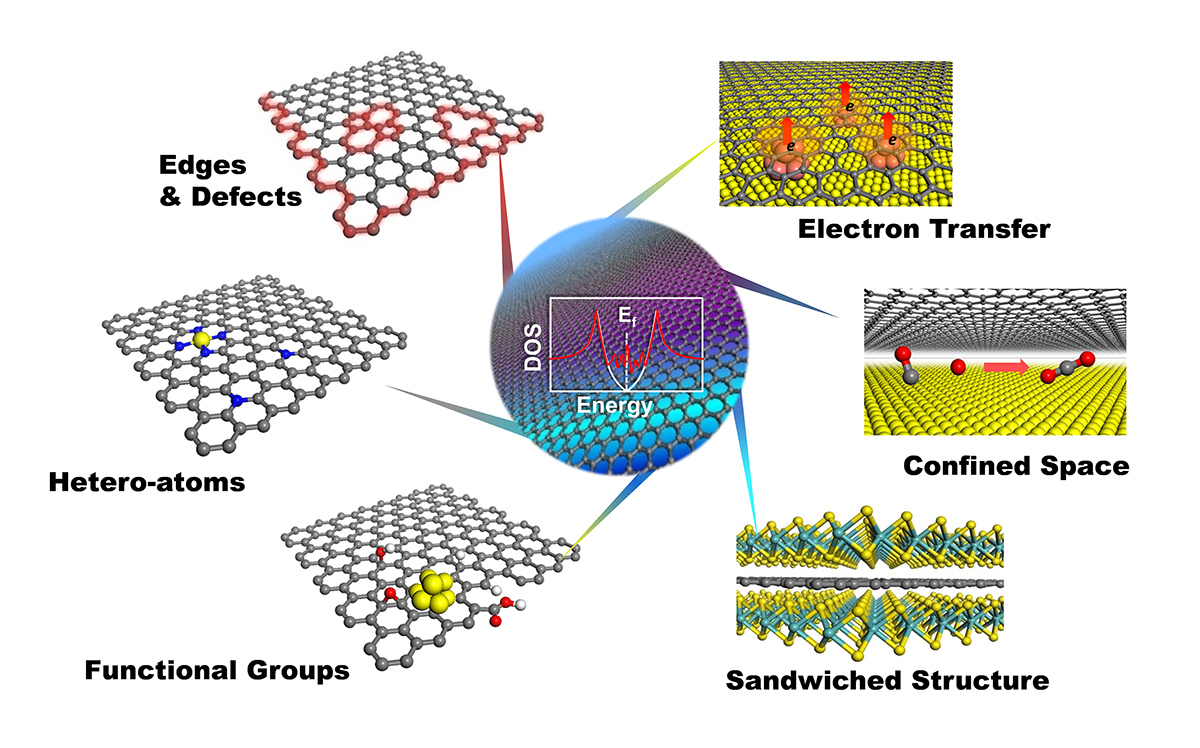
Graphene possesses high stability under harsh reaction conditions, unique electronic structure near the Fermi level, and tunable catalytic reactivity for chemical reactions. Thus, combining graphene with highly active non-noble metal nanoparticles to form a core-shell structure, with metal nanoparticles fully encapsulated by graphene (or carbon nanotube) layers, we obtain the 'Chainmail' catalyst. The metallic cores are fully protected by the graphene 'armor' against the etching reaction environment. Meanwhile they can induce reactivity on the graphene surface via electronic coupling with the carbon atoms and transfer of d electrons to the outer surface of the graphene. The activity of 'Chainmail' catalyst depends on a variety of factors including composition of metal cores, thickness and curvature of the graphene layers, doping of hetero-atoms into the graphene lattice, and graphene lattice defects, etc., which will be further elucidated in the project. Application of 'Chainmail' catalyst in energy catalysis systems will also be extended.
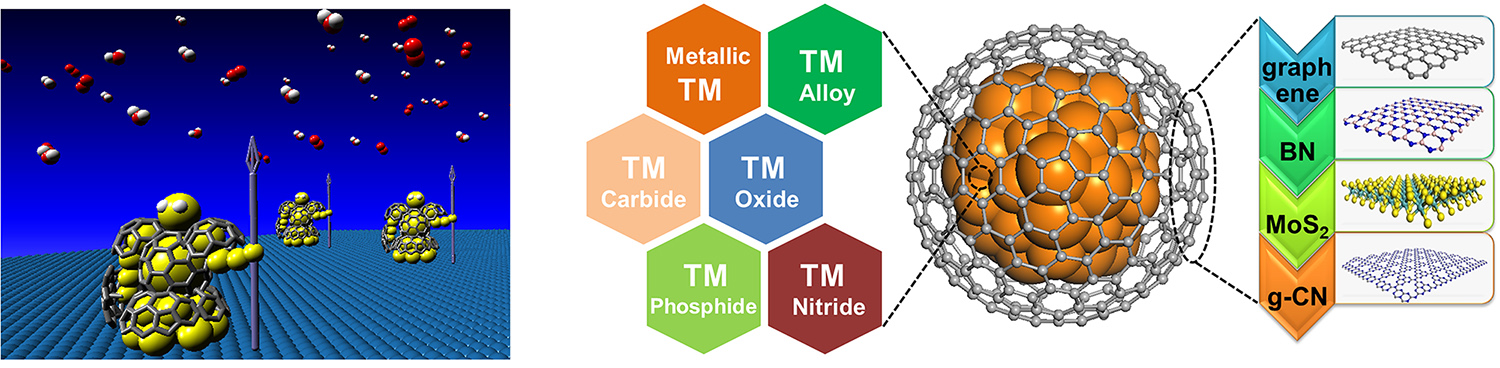
◆ 2D materials confining single atom moieties
Metal-N4 confined in graphene lattice provides coordinatively unsaturated metal center that delivers unique activity in various catalytic processes, such as low-temperature benzene oxidation (Science Advances, 2015, 1, e1500462), oxygen reduction reaction (Nano Energy, 2017, 32, 353-358), and low-temperature methane oxidation (Chem, 2018, 4, 1-9), etc. We are exploring applications of the structure in more catalytic systems aiming at highly efficient conversion of energy molecules. Replacing the S or Mo atom or both in 2D MoS2 with other moieties brings new opportunities of inducing catalytic activities on the basal plane S atoms. We are systematically studying the effect of hetero-atom doping on modulating the activity of MoS2 toward energy catalysis.