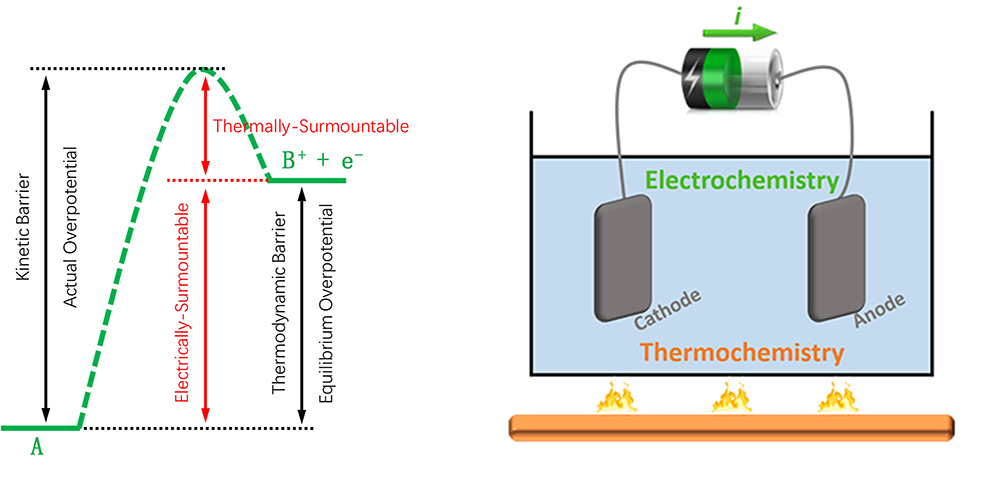
Electrochemical catalysis is a key technology that realizes efficient conversion between electrical and chemical energy in many renewable energy storage and utilization devices. In an electrochemical reaction process that is driven by electric potential, the elementary steps that involve transfer of electrons are favored by the electrical work on electrons, however, for those not involving transfer of an electron, the electric potential has no effect on the reaction thermodynamics and they can only resort to thermal energy to overcome the thermodynamic barriers. Thermal activation is also helpful to decrease the overpotential that originates from the kinetic barrier part higher than the thermodynamic barrier. Thus, we are exploring routes of coupling thermochemical and electrochemical catalysis to promote the conversion of highly stable molecules, such as CH4, CO, and N2, to value-added products with high efficiency.