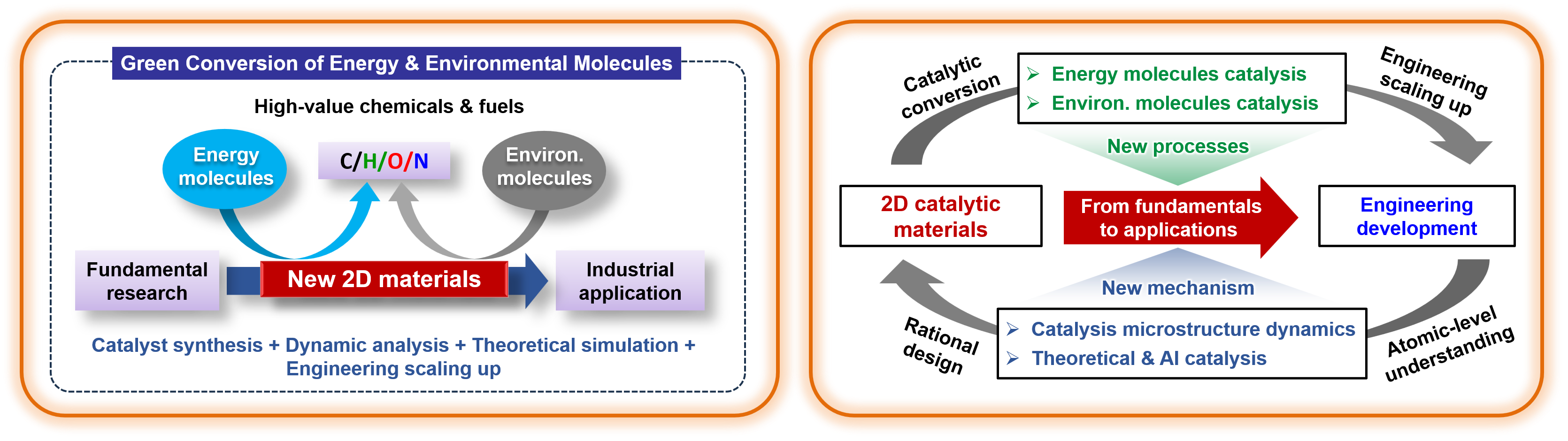
Energy & Environmental Catalysis(EEC)group is a unit of the State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, headed by Prof. Dehui Deng. Our research focus on the development of 2D material-based catalyst and their applications in catalytic conversion of energy and environmental molecules, mainly including:
◆ Design and synthesis of advanced 2D materials and their heterostructures, including graphene, MoS2, LDHs et al., and modulating their surfaces and interfaces to trigger efficient catalytic reactivities.
◆ Thermochemical, electrochemical, or thermo-electrical coupled catalysis toward highly efficient activation and conversion of energy molecules, including O2, H2, H2O, CO, CO2, CH4 and CH3OH.
◆ Computational and theoretical modeling of energy catalysis systems to provide fundamental understandings of catalytic activition and reaction mechanism for catalyst optimization and development.
Subgroups
531 Group:2D catalytic materials(二维催化材料研究组)
532 Group:Energy molecules catalysis, Group leader: Assoc.Prof. Xiaoju Cui(能源小分子催化研究组,组长:崔晓菊 副研究员)
533 Group:Environmental molecules catalysis(环境小分子催化研究组,组长:黄瑞 研究员)
534 Group:Catalysis microstructure dynamics, Group leader: Prof. Wei Liu(催化动态解析组,组长:刘伟 研究员)
535 Group:Theoretical & AI catalysis, Group leader: Assoc.Prof. Liang Yu(理论与智能催化组,组长:于良 研究员)
536 Group:Engineering development, Group leader: Assoc.Prof. Yanting Liu(工程化开发组,组长:刘艳廷 副研究员)
On-going Projects
1. 'Chainmail' catalysts
Graphene possesses high stability under harsh reaction conditions, unique electronic structure near the Fermi level, and tunable catalytic reactivity for chemical reactions. Thus, combining graphene with highly active non-noble metal nanoparticles to form a core-shell structure, with metal nanoparticles fully encapsulated by graphene (or carbon nanotube) layers, we obtain the 'Chainmail' catalyst. The metallic cores are fully protected by the graphene 'armor' against the etching reaction environment. Meanwhile they can induce reactivity on the graphene surface via electronic coupling with the carbon atoms and transfer of d electrons to the outer surface of the graphene. The activity of 'Chainmail' catalyst depends on a variety of factors including composition of metal cores, thickness and curvature of the graphene layer, doping of hetero-atoms into the graphene lattice, and graphene lattice defects, etc., which will be further elucidated in the project. Application of 'Chainmail' catalyst in energy catalysis systems will also be extended.
(Advanced Materials, 2017, 29, 1606967; Nature Nanotechnology, 2016, 11, 218-230;Energy & Environmental Science, 2016, 9, 123-129; Angewandte Chemie International Edition, 2015, 54, 2100-2104; Energy & Environmental Science, 2014, 7, 1919-1923; Angewandte Chemie International Edition, 2013, 52, 371-375)
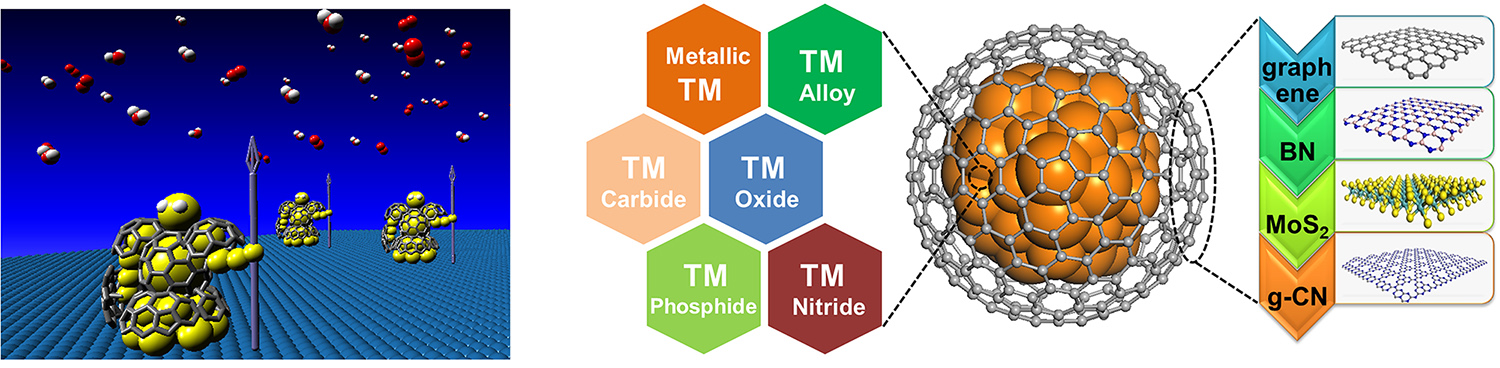
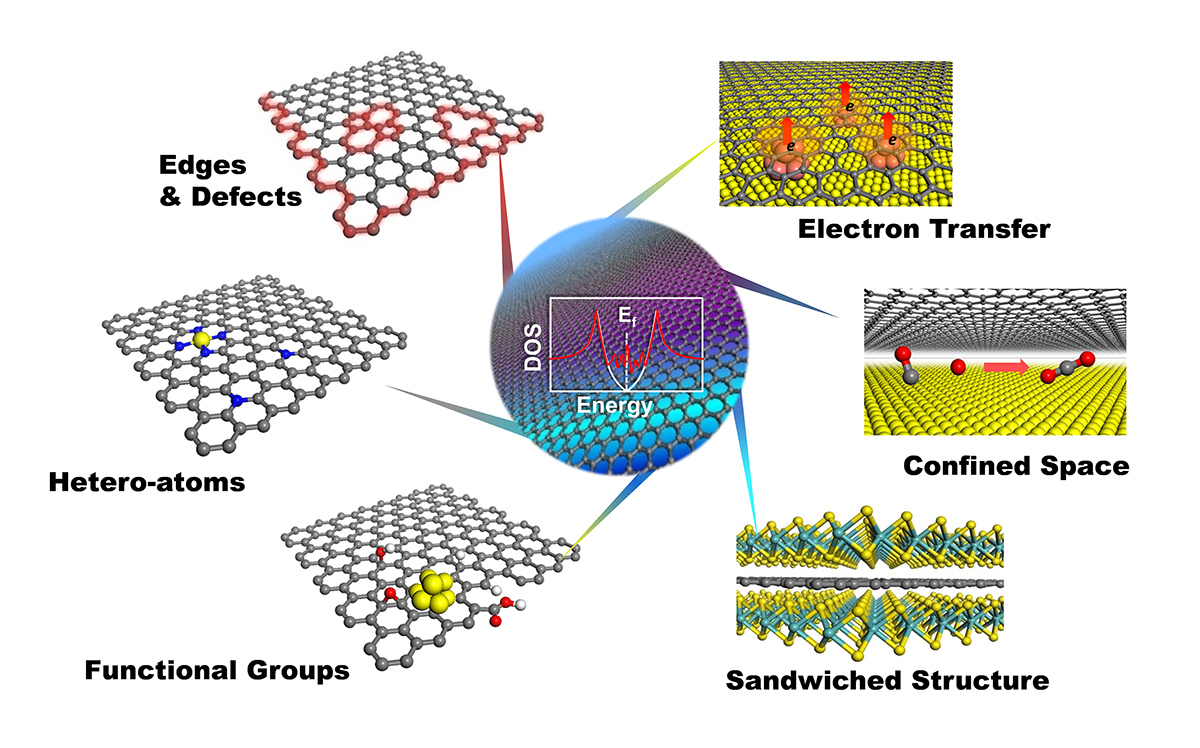
2. 2D materials confining single atoms
Metal-N4 confined in graphene lattice provides coordinatively unsaturated metal center that delivers unique activity in various catalytic processes, such as low-temperature benzene oxidation, oxygen reduction reaction, and low-temperature methane oxidation, etc. We are exploring applications of the structure in more catalytic systems aiming at highly efficient conversion of energy molecules. Replacing the S or Mo atom or both in 2D MoS2 with other moieties brings new opportunities of inducing catalytic activities on the basal plane S atoms. We are systematically studying the effect of hetero-atom doping on modulating the activity of MoS2 toward energy catalysis.
(Chem, 2018, 4, 1-9; Nature Communications, 2018, 9, 1181; Nature Communications, 2017, 8, 14430; Nano Energy, 2017, 32, 353-358; Angewandte Chemie International Edition, 2016, 55, 6708-6712; Energy & Environmental Science, 2015, 8, 1594-1601; Science Advances, 2015, 1, e1500462)
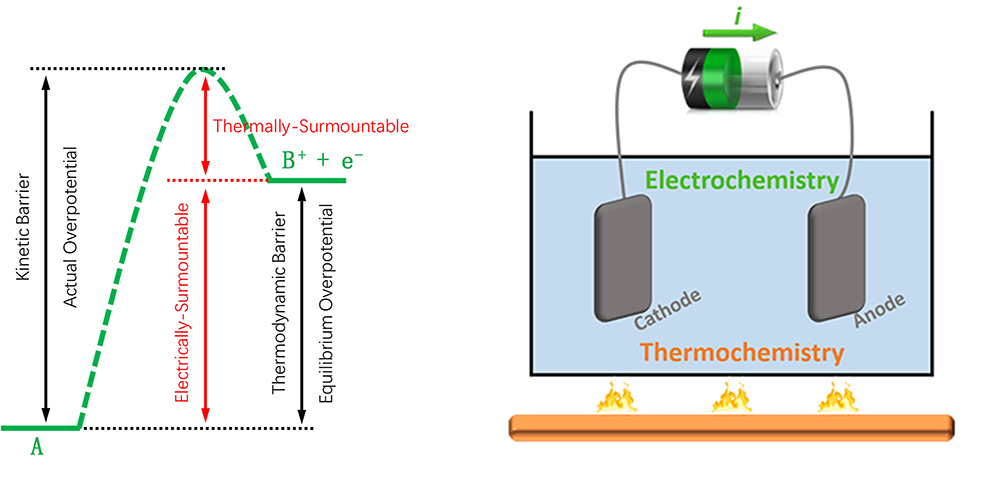
3. Thermo-electrical coupled catalysis for activation and conversion of energy molecules
Electrochemical catalysis is a key technology that realizes efficient conversion between electrical and chemical energy in many renewable energy storage and utilization devices. In an electrochemical reaction process that is driven by electric potential, the elementary steps that involve transfer of electrons are favored by the electrical work on electrons, however, for those not involving transfer of an electron, the electric potential has no effect on the reaction thermodynamics and they can only resort to thermal energy to overcome the thermodynamic barriers. Thermal activation is also helpful to decrease the overpotential that originates from the kinetic barrier part higher than the thermodynamic barrier. Thus, we are exploring routes of coupling thermochemical and electrochemical catalysis to promote the conversion of highly stable molecules, such as CH4, CO, and N2, to value-added products with high efficiency.
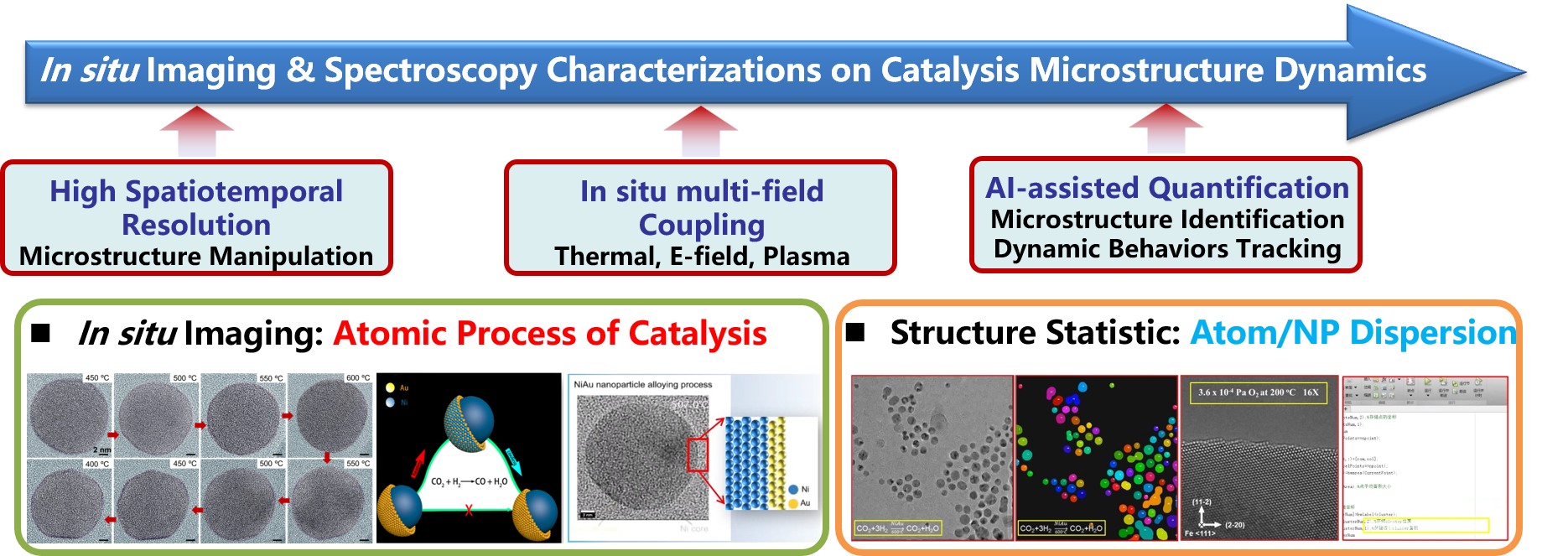
4. In situ Imaging & Spectroscopy Characterizations on Catalysis Microstructure Dynamics
A working catalyst contains microstructure diversity that susceptible to change upon the stimuli of undergoing reaction. This dynamically restructured surface accounts for catalytic activity, facilitating real active sites. Visualizing these catalyst reconstructions and detecting their interactions with reactant molecules and intermediates in spatiotemporal consistency (real time at the same location) are of vital importance through combined using cutting-edge techniques of atom-resolved in situ microscopy and spectroscopy, e.g. in situ S/TEM, FTIR, Raman. Research interests could be divided into three perspectives: (1) atomic structure evolution in 2D materials catalysis; (2) AI-based catalyst microstructure analysis and instrument development for operando microscopy-spectroscopy combined characterizations. Stemming from these advanced characterization technologies, insights into the microscale origins of catalytic activity have been unraveled, including in situ tracking the bimetallic nanocatalyst reconstructions to identify active structures(Nat. Catal. 2020, 3, 411-417; ChemCatChem 2024, e202400085; J. Am. Chem. Soc. 2023,145, 22671-22684; ACS Catal. 2022, 12, 10531-10545); reaction-induced carbides formation and their C-C coupling performances (Adv. Mater. 2024, 2404046; Small Methods 2022, 6, 2270036; Carbon 2017, 117, 192-200); discerning active sites via metal dispersion analysis and quantified atom/particle-counting from TEM imaging (Angew. Chem. Int. Edit. 2022, 61, e202214166; J. Am. Chem. Soc. 2022,144, 12062-12071; J. Am. Chem. Soc. 2021, 143, 15243-15249)
5. Computational and theoretical modeling of energy catalysis processes
Rational design and optimization of catalyst toward high reactivity and selectivity requires clear understanding of the activation mechanism, reaction mechanism, and the underlying factors that determine the reaction thermodynamics and micro-kinetics at an atomic level. This is rather challenging due to the complexity in catalyst structure and composition and the reaction network, and the limited information that is obtained from experimental characterizations. Here, we employ theoretical and computational chemistry tools to simulate catalytic conversion of energy molecules and obtain the key information about the reaction systems, i.e. the active centers, reaction mechanisms, and structure-reactivity relationships, etc., aiming to provide insights into the nature of 2D materials in catalytic conversion of energy molecules for rational design and screening of 2D material catalysts.
(Nano Energy, 2017, 32, 353-358; Science Advances, 2015, 1, e1500462; Angewandte Chemie International Edition, 2013, 52, 371-375)
