Recently, Prof. Dehui Deng from SKLC, DICP collaborated with Prof. Ye Wang and Prof. Jun Cheng from Xiamen University to get a great progress in direct synthesis of ethylene glycol via selective C-C coupling of methanol. This work has been published in Nature Communications (Nature Communications).
Selective transformation of methanol into C2 or multi-carbon molecules via controlled C−C coupling is challenging and attractive target. Current conversion of methanol involving C−C bond formation are restricted to dehydrative oligomerizations such as methanol-to-olefin and methanol-to-gasoline processes, which show limited selectivity to a specific product. The preferential activation of inert C-H bond in methanol without affecting the hydroxyl group to produce ethylene glycol is one of the most challenge reactions in chemistry.
As an important basic chemical, ethylene glycol is not only used as the main feedstock in synthesis of polyethylene terephthalate (PET), but also has other wide applications in chemical industry. Currently, more than 90% of ethylene glycol is produced by petroleum-derived ethylene via epoxidation and subsequent hydrolysis of ethylene epoxide, leading to a low-efficient and high energy consumption process. Also, the synthesis of ethylene glycol via dimethyl oxalate from coal-based syngas is a long technological and high-cost process. The methanol can be derived from a variety of carbon resources, such as natural gas or shale gas, coal, biomass, and carbon dioxide, and is an abundant and renewable C1 building block. Therefore, the direct synthesis of ethylene glycol from methanol is of great significance.
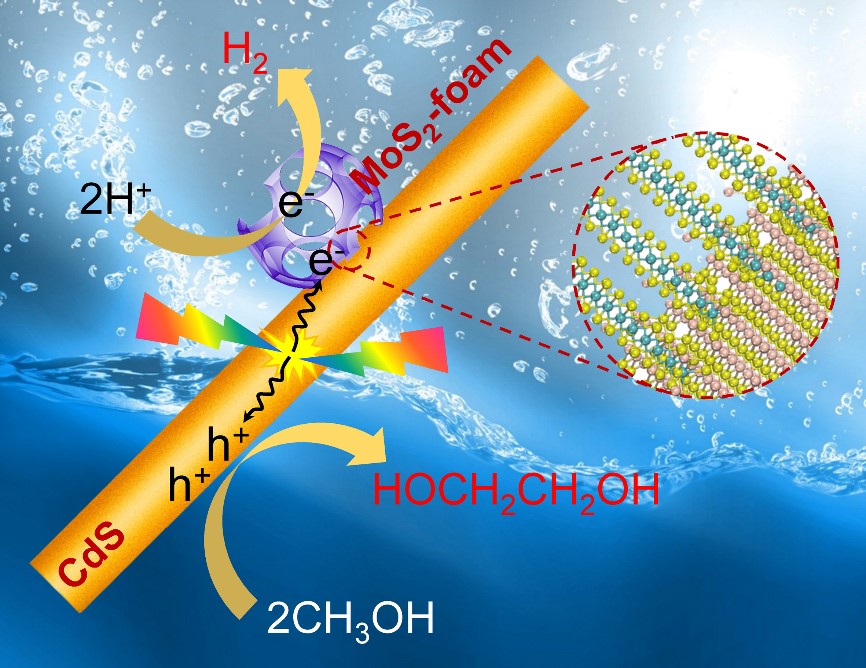
On the basis of long-time for studying of two-dimensional MoS2 catalytic materials (Nat. Commun.; Nat. Nanotechnol.; Energy Environ. Sci.), Prof. Dehui Deng’s group with Prof. Ye Wang’s and Prof. Jun Cheng’s groups has for the first time constructed a structure of mesoporous MoS2 nanofoam modified CdS nanorod, which shows a high performance in direct synthesis of ethylene glycol via C-C coupling of methanol under visible light. By designing a process-intensified reactor with ethylene glycol separation during the reaction, the selectivity, yield and quantum efficiency (450 nm) can reach 90%, 16%, and 5%, respectively. The DFT calculations suggest that the cleavage of C−H bond in CH3OH on CdS surfaces occurs through a concerted proton-electron transfer (CPET) mechanism driven by the photogenerated hole, producing •CH2OH radical with a low activation barrier. The weakly adsorbed •CH2OH can readily desorb from CdS surfaces. MoS2-foam can not only effectively promote the transfer of electrons on the surface of CdS, and generate photogenerated holes, but also promote the coupling of •CH2OH into ethylene glycol in MoS2-foam channels. The prepared MoS2-foam/CdS composite catalyst greatly promoted the formation rate of ethylene glycol, and the formation rate of ethylene glycol was over 20 times higher than that of pure CdS. The present visible light-driven methanol conversion not only offers a high-efficient approach for ethylene glycol synthesis under moderate condition, but also opens up an avenue for designing new reactions, in particular those involving preferential C-H bond activation without affecting other functional groups in the same molecule.
This work is supported by Ministry of Science and Technology of China, National Natural Science Foundation of China, Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, and Collaborative Innovation Center of Chemistry for Energy Materials. (2011•iChEM)
Link:http://www.dicp.cas.cn/xwdt/kyjz/201811/t20181119_5187802.html