Recently, research group led by Prof. Dehui Deng from the State Key Laboratory of Catalysis (SKLC), Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, reviewed the latest progress about membrane electrode assembly (MEA) for electrocatalytic carbon dioxide (CO2) reduction, especially focusing on the working principle and application examples. This work has been published in Angewandte Chemie International Edition.
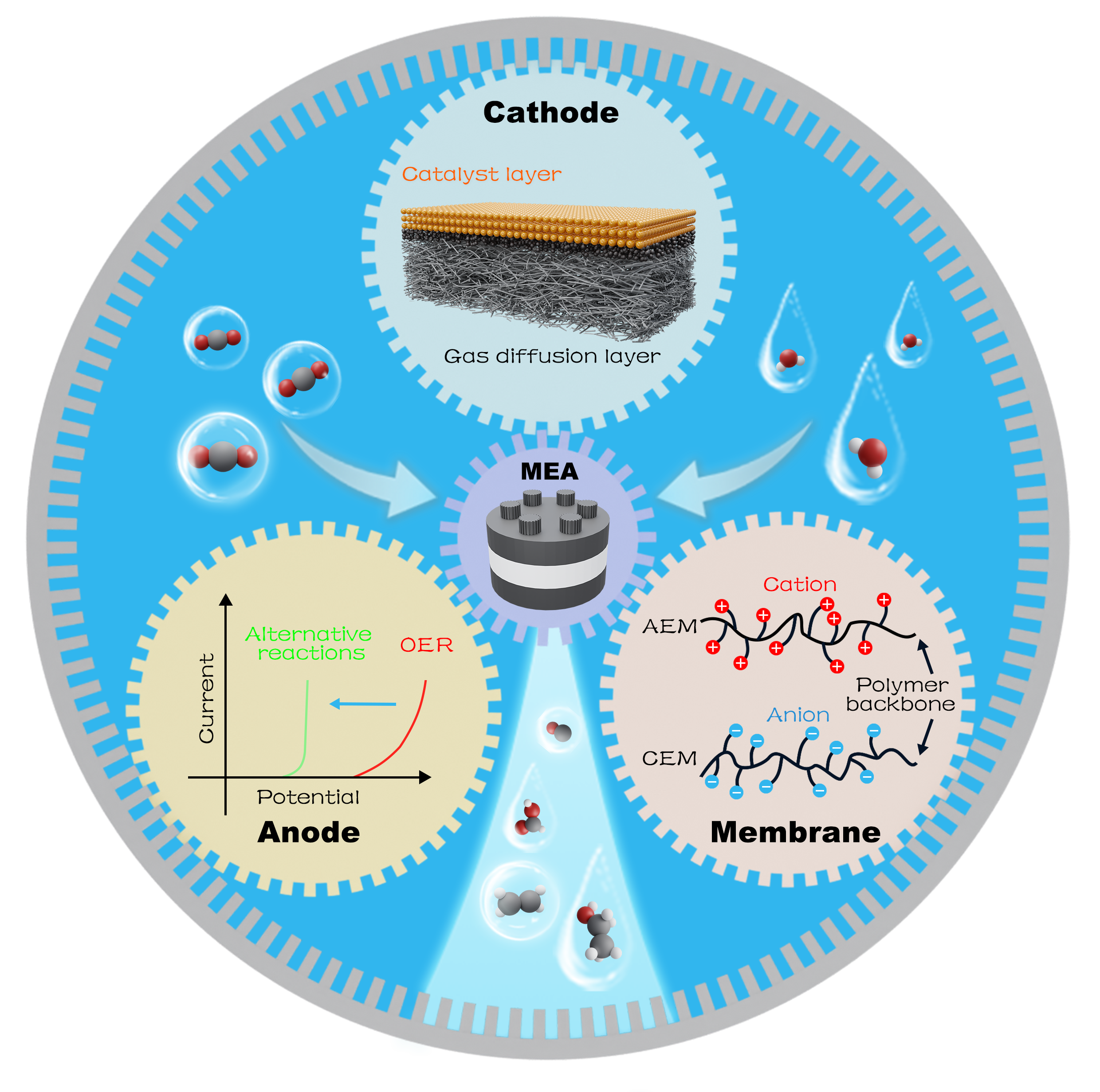
Schematic illustration of the interaction over cathode, membrane and anode to improve MEA’s electrocatalytic efficiency.
The excessive emission of CO2 has caused serious environmental issues. Electrocatalytic CO2 reduction for the generation of high value-added products can not only solve the environmental issues from CO2 emission, but also store the intermittent electricity into easily transportable chemicals. Currently, MEA is a kind of technique that can convert CO2 with high efficiency and low energy consumption. There are several characteristics for this technique: 1) gas CO2 can be directly transferred to cathode’s interface; 2) only one layer of ion exchange membrane exists between cathode and anode; 3) anolyte flows through outside circulation. Above all, MEA would be developed as a technique to promote CO2 conversion at industrial scale.
This review firstly discussed the characteristics and working principles of each component over MEA electrolyser, including gas diffusion electrode, ion exchange membranes, alternative anode reactions. Then voltage distribution of each component during reaction was scrutinized. After that, the application of MEA for the generation of various products from CO2RR was summarized, highlighting the research progress. Finally, the challenges of this promising technique and the perspective on this direction were summed up for future research.
Prof. Dehui Deng’s group has accomplished a series of research progress on C1 catalysis field, including low-temperature CO2 hydrogenation to methanol (Nat. Catal., 2021), eletrocatalytic CO2 reduction (Angew. Chem. Int. Ed., 2018;Cell Rep. Phys. Sci., 2020; J. Energy Chem., 2022; Nano Res., 2019), low-temperature methane conversion to oxygenates (Chem, 2018; Chem, 2019; Nano Energy, 2021; Chem Catal., 2022), room-temperature electrochemical water-gas shift reaction for high purity hydrogen production (Nat. Commun., 2019; Nano Energy, 2022), electrocatalytic CO reduction for ethylene generation (Angew. Chem. Int. Ed., 2020), visible-light-driven dehydrogenative coupling of methanol to ethylene glycol (Nat. Commun., 2018). These works have provided important references for C1 catalysis field.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences.
Link: http://www.dicp.cas.cn/xwdt/kyjz/202304/t20230412_6733393.html