A research group led by Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. ZHANG Jianan from Zhengzhou University, reported a synergetic effect of atomic-interface from well-constructed 2H-MoS2/Fe(SA)-N-C by assembling 2H-MoS2 layer on N-doped carbon confined Fe atom as anode material, which boosts the reversible sodium storage capacity.
This study was published in Angewandte Chemie International Edition on Feb. 02.
Regulating the catalytic process by atomic-interface for enhanced sodium ion storage (Image by XIA Huicong and ZAN Lingxing)
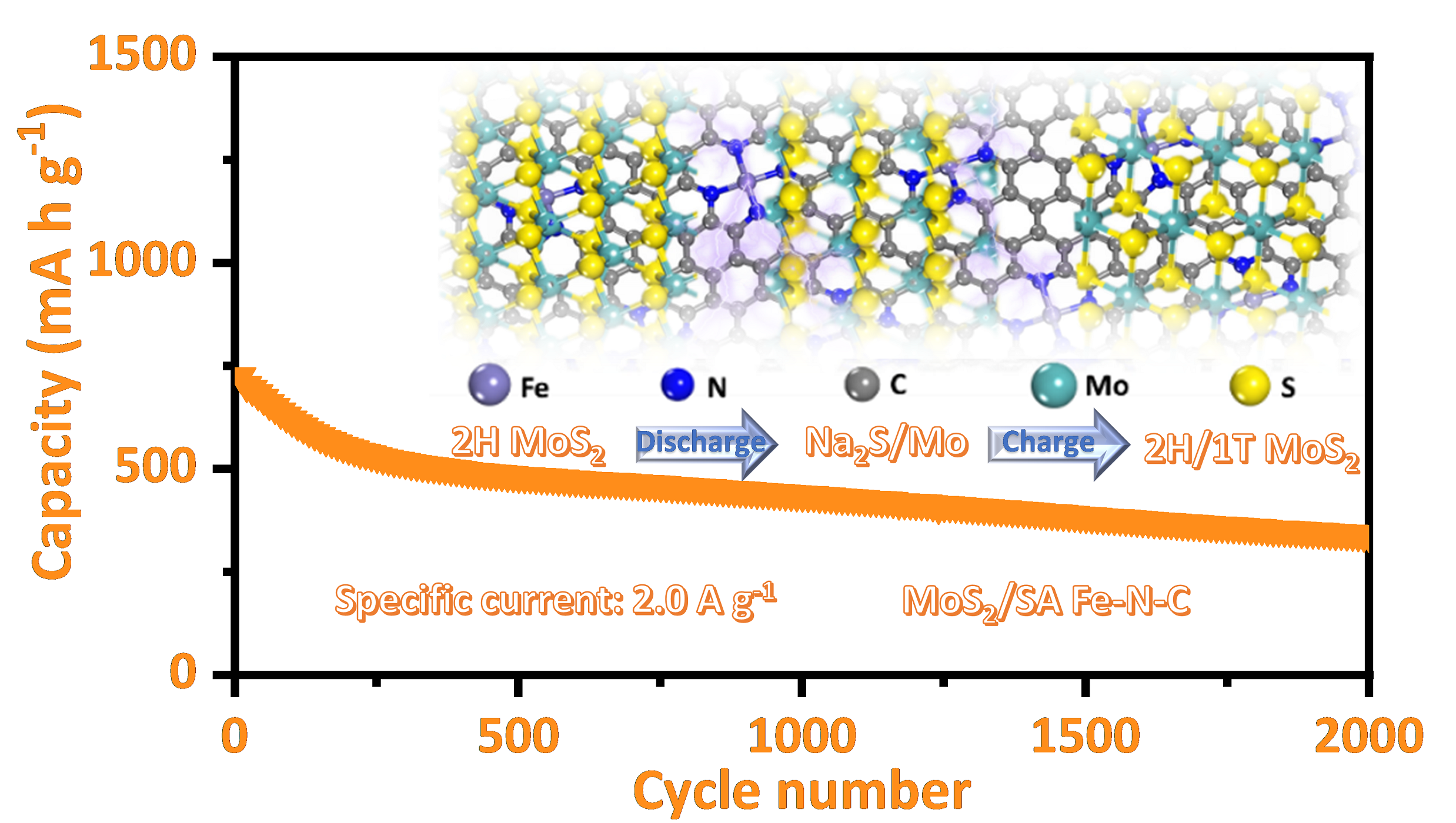
The researchers assembled 2H-MoS2 nanosheets on Fe(SA)-N-C through interfacial chemistry engineering as the anode material for sodium ion storage. They found that driven by work function variance, at heterointerface, the electron could transfer from Fe(SA)-N-C to 2H-MoS2 easily, enhancing the adsorption Na+ ion at the S sites of electron-rich MoS2.
Meanwhile, they also indicated that the spin-state of Fe site of Fe(SA)-N-C changed, which proved that electronic structure and catalytic activity of the Fe site were optimized.
Compared with nitrogen-doped carbon (N-C) and pure carbon, the Fe sites of Fe(SA)-N-C could effectively promote the phase transition and structural evolution of 1T/2H-MoS2 during the sodiation/desodiation process, thus realizing to prolong its cycling life. Therefore, 2H-MoS2/Fe(SA)-N-C showed a good long-term cycling performance with a capacity of 350 mA h g-1 after 2000 cycles at 2.0 A g-1.
This work was supported by the National Natural Science Foundation of China, the Strategic Priority Research Program of CAS, and the Danish company Haldor Topsøe A/S.
Link: http://www.dicp.ac.cn/xwdt/kyjz/202302/t20230227_6683443.html