Recently, a research group led by Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) designed a catalyst of PdCu nanoparticles supported on carbon nanotubes (Pd0.7Cu/CNTs) to boost hydrogen production from RT-EWGS, which significantly enhanced the oxidation activity of CO at the anode and improved the hydrogen production efficiency at the cathode.
This study was published in Nano energy (Nano Energy 2022, 102, 107704).
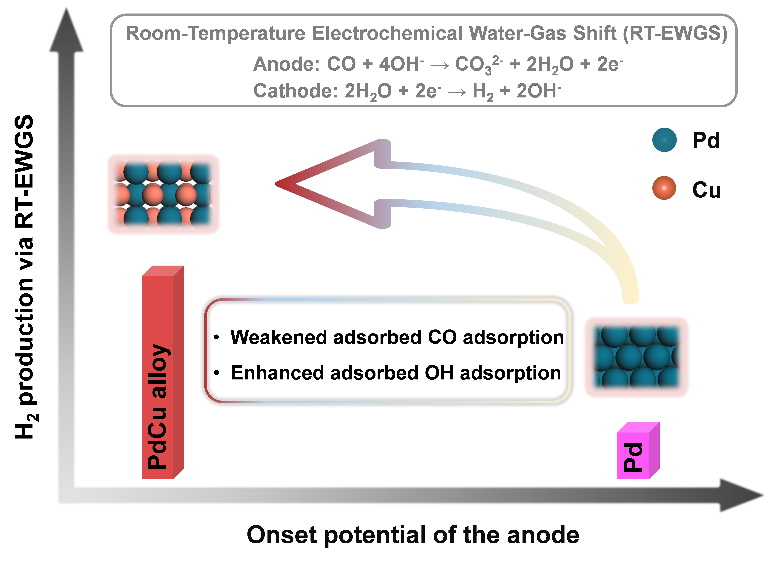
PdCu alloy boosts hydrogen production via room-temperature electrochemical water-gas shift reaction(Image by WEI Huifang)
Room-temperature electrochemical water-gas shift (RT-EWGS) process is recently proposed as a novel direction for hydrogen production under ambient conditions by Deng in 2019, in which the water-gas shift redox reaction is electrochemically decoupled to separated cathodic hydrogen evolution reaction and anodic carbon monoxide (CO) oxidation reaction. The hydrogen from RT-EWGS is of high purity over 99.99%, which can be used directly without complex separations. While the anodic CO oxidation as the key and bottleneck-type reaction largely hinders the overall efficiency due to its sluggish reaction kinetics. It is of great significance to develop low cost and efficient anode electrocatalysts to enhance the hydrogen production via improving the CO oxidation activity, but it remains a great challenge.
In this study, by alloying Pd with Cu, the researchers developed the catalyst of PdCu nanoparticles supported on carbon nanotubes (Pd0.7Cu/CNTs) to boost hydrogen production from RT-EWGS.
The mass activity can reach 19.9 mA/mgPd for anodic CO oxidation at 0.3 versus reversible hydrogen electrode (vs. RHE), which is over 330 times higher than that pure Pd nanoparticles supported on carbon nanotubes. Moreover, the optimized Pd0.7Cu/CNTs catalyst exhibits a record activity, which delivers a mass activity nearly 5 times higher than that over the previously reported Pt2.7Cu catalyst at 0.2 V vs. RHE.
Combined with the density functional theory calculations, we find that the adsorbed CO (CO*) species is more likely to react with the adsorbed OH (OH*) rather than the OH- in the solution for PdCu alloy catalyst during anodic CO oxidation process, and the introduction of Cu into Pd renders a weakened CO* adsorption along with an enhanced OH* adsorption, which significantly lower the overpotential via optimizing the anodic oxidation of CO pathway.
This study provides a new direction for the design of efficient anode catalysts toward RT-EWGS.
The above work was supported by the National Natural Science Foundation of China, Strategic Priority Research Program of the Chinese Academy of Science, Innovation Research Fund Project of DICP, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM). (Image and text by WEI Huifang and CUI Xiaoju)
Link: http://www.dicp.cas.cn/xwdt/kyjz/202209/t20220928_6518207.html