Methane, as the main component of shale gas, natural gas and combustible ice, is among the most promising energy resources for producing high-value chemicals. However, it is still challenging to activate methane under mild conditions due to the high symmetry and low polarizability of methane molecule.
Recently, a research group led by Prof. DENG Dehui and Assoc. Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved highly efficient room-temperature methane conversion to liquid C1 oxygenates over ultrathin two-dimensional (2D) Ru nanosheets (NSs) with lattice-confined Cu atoms.
This study was published in Chem Catalysis on August 24.
Conversion of methane to C1 oxygenates at the edge-confined Cu sites of Ru nanosheets. (Image by FAN Jinchang)
Ultrathin 2D metallic NSs are promising matrix materials for creating active centers for the methane activation by confining heteroatoms in the lattice. However, the hardly controllable tailoring of the coordination environment for the confined heteroatoms in the 2D NSs makes it a challenge for the construction of effective active sites for methane activation.
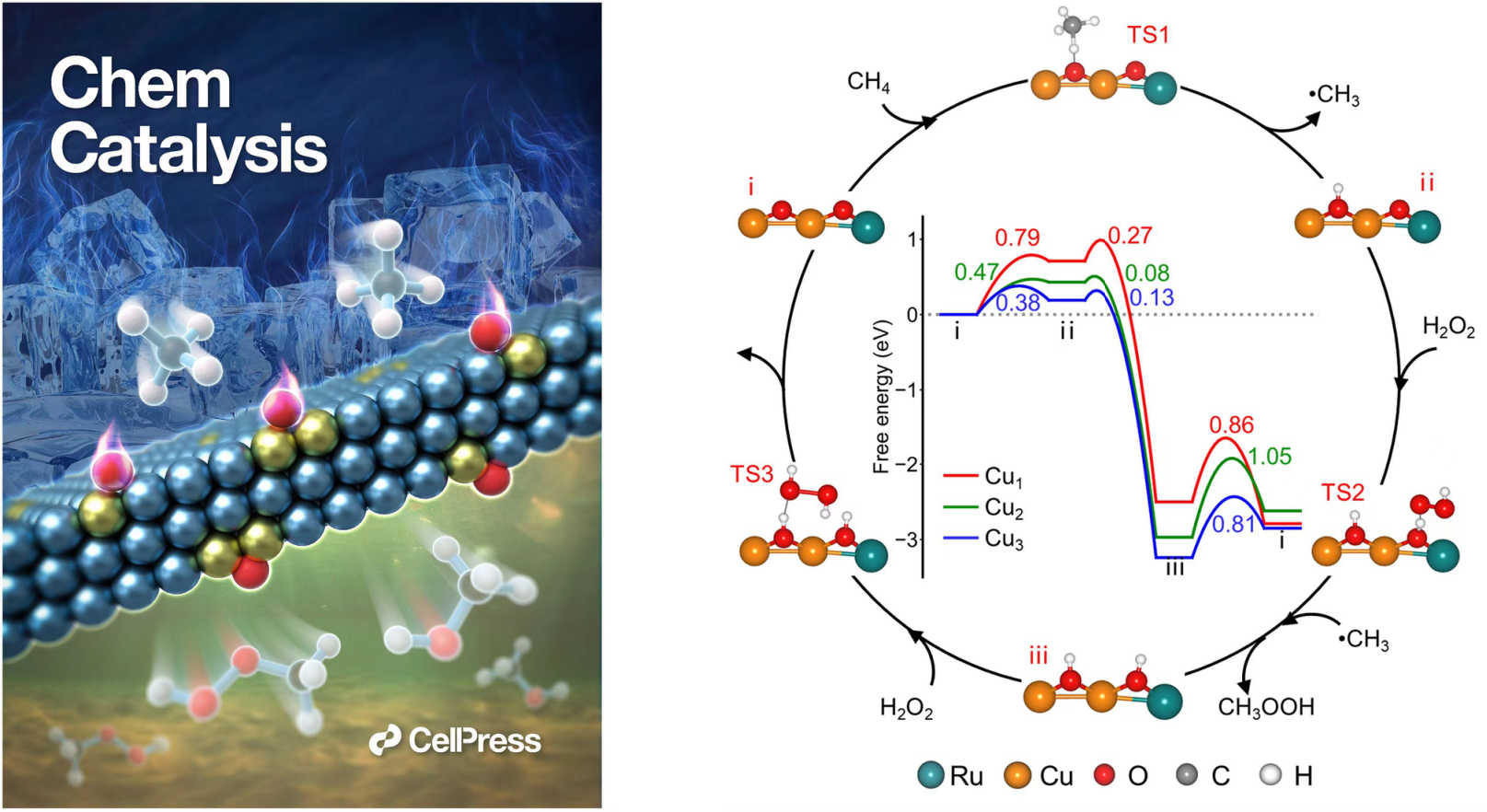
In this study, the researchers developed the catalysts by confining Cu atoms in ultrathin 2D metallic Ru NSs through a unique strategy of noble metal-induced reduction mechanism, which enabled a highly selective methane conversion to liquid C1 oxygenates under room temperature.
By precisely adjusting the content of the confined Cu atoms to optimize their coordination environment, they achieved the productivity of liquid C1 oxygenates (CH3OOH and CH3OH) over the Ru11Cu catalyst to a maximum of 1533 mmol g-1Cu(surf.) h-1 with an over 99% selectivity using H2O2 as the oxidant.
Multiple spectroscopic analysis and first-principles calculations revealed that bi-coordinated bridge-site oxygen species generated on the Ru edge-confined Cu sites could facilely dissociate the C-H bond of methane with a moderately low energy barrier, and thus enabled the methane conversion at room temperature via a free radical mechanism.
"This study provides a strategy for designing efficient catalysts by constructing edge-confined active centers in metallic NSs for the activation of C-H bonds in light alkanes," said Prof. DENG.
The above work was supported by the National Natural Science Foundation of China, the Key Research Program of Frontier Sciences of CAS, and the China Postdoctoral Science Foundation. (Text by FAN Jinchang and YU Liang)
Link: http://www.dicp.cas.cn/xwdt/kyjz/202208/t20220830_6505555.html