Research group led by Prof. Dehui Deng from the State Key Laboratory of Catalysis (SKLC), Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, has attracted much attention due to their efforts on the catalytic conversion of C1 energy molecules under mild conditions. Recently, they were invited to publish a review article entitled "Catalytic Conversion of C1 Molecules under Mild Conditions".
The catalytic conversion of C1 molecules (methane, carbon monoxide, methanol, carbon dioxide, etc.) plays an important role in the conversion and utilization of carbon-based energy such as coal, natural gas, and biomass. In order to improve the conversion efficiency, the current industrial processes are usually operated under harsh reaction conditions such as high temperature and high pressure, but this also leads to an increase in reaction energy consumption and the production of unnecessary by-products. The catalytic conversion of C1 molecules under mild conditions can not only reduce the energy consumption of the reaction, but also render a better control the selectivity of the target products, which has always been the goal pursued by academia and industry. This review provides a systematic overview of the recent research progress in the conversion of C1 molecules to high-quality chemicals and fuels under low-temperature thermal catalysis, electrocatalysis, and photocatalysis under mild conditions. It focuses on the catalyst synthesis, reactor design, energy input to the reaction process and mechanism understanding and so on. In addition, the prospect is offered to highlight the challenges and future research directions towards C1 molecules conversion under mild conditions.
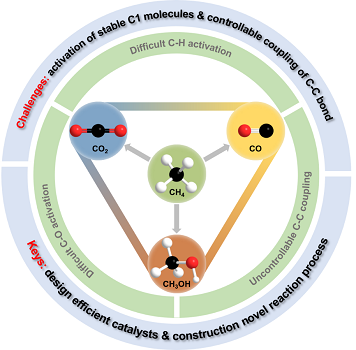
Caption: The keys and challenges for mild conversion of C1 energy molecules
Prof. Dehui Deng etc. from SKLC, DICP have accomplished several pioneered work in C1 catalysis field. As representative researches, they for the first time reported room-temperature electrochemical water-gas shift reaction for high purity hydrogen production (Nat. Commun. 2018, 9, 1181). They also for the first time achieved visible-light-driven dehydrogenative coupling of methanol to ethylene glycol (Nat. Commun. 2019, 10, 86). They developed low-temperature methane conversion to C1 oxygenates (Chem 2018, 4, 1902; Chem 2019, 5, 2296; Nano Energy, 2020, in press). In addition, they developed highly selective production of ethylene by electroreduction of carbon monoxide (Angew. Chem. Int. Ed. 2020, 59, 154) and efficient electrocatalytic carbon dioxide reduction to carbon monoxide (Angew. Chem. Int. Ed. 2018, 57, 16339; Cell Rep. Phys. Sci. 2020, 1, 100145; Nano Res. 2019, 12, 2313). These works have received wide attentions in the world.
This work has recently been published in EnergyChem. This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, the DNL Cooperation Fund of Chinese Academy of Sciences. (Text by Xiaoju Cui and Hehua Gao)
Link: http://www.dicp.cas.cn/xwdt/kyjz/202012/t20201224_5839075.html