Acetylene hydrogenation to ethylene (AHE) in combination with acetylene production from coal pyrolysis, is considered as a promising alternative to the traditional petroleum-based ethylene production route. However, thermocatalytic AHE (T-AHE) process often suffers from high temperatures (100-300 oC) and high consumption of molecular hydrogen (H2).
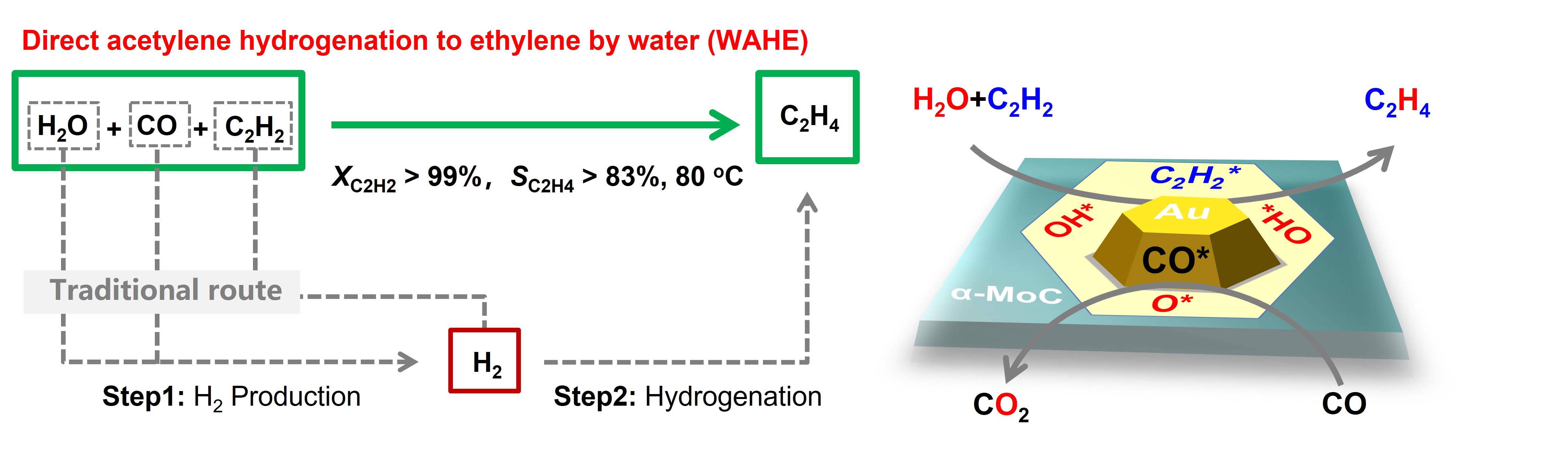
Recently, a research group led by Prof. DENG Dehui and Assoc. Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) realized a new process of acetylene hydrogenation to ethylene by directly using water as hydrogen source (WAHE) and low-cost CO as oxygen acceptor over a Au/α-MoC catalyst.
This study was published in Nature Catalysis on September 28.
Acetylene hydrogenation to ethylene by water at low temperature on a Au/α-MoC catalyst (Image by Rui Huang)
The hydrogenation of acetylene can proceed by a H2-based thermocatalytic route or H2O-based electrocatalytic route. The thermocatalytic AHE is energy demanding due to the requirement of relatively high temperature and H2 consumption, since H2 is also industrially produced by energy-intensive processes such as steam methane reforming (SMR) and water gas shift (WGS) reaction. The electrochemical AHE (E-AHE) process is advantageous as it directly uses electrochemically-generated active hydrogen species from water to hydrogenate acetylene without involving the unnecessary formation of H2. Integrating this concept into the T-AHE process would be very attractive but so far has never been achieved.
The WAHE process delivers over 99% acetylene conversion and high ethylene selectivity of 83% at 80 oC. Mechanistic studies reveal that in-situ generated hydroxyl species from water dissociation at the boundary of Au and α-MoC, serving as mild reductants, enables the selective semi-hydrogenation reaction with residual O removed by CO. This process circumvents H2 consumption in the classical route and opens avenues for energy-efficient acetylene hydrogenation by water at low temperature.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China and the Strategic Priority Research Program of CAS.
Link:
https://www.nature.com/articles/s41929-023-01026-y
http://www.dicp.ac.cn/xwdt/ttxw/202310/t20231007_6890090.html