Direct conversion of methane (CH4) to high value-added chemicals at room temperature, by directly using abundant and low-cost molecular oxygen (O2) as oxidant, is a dream reaction for chemists to establish an energy-efficient and sustainable route for the CH4 utilization, but it remains a great challenge owing to the chemical inertness of methane and low activity of O2.
Recently, a research group led by Prof. DENG Dehui and Assoc. Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), for the first time report direct CH4 conversion to C1 oxygenates (CH3OH, HOCH2OH and HCOOH) by directly using O2 at room temperature (25 oC) over an edge-rich MoS2 catalyst.
This study was published in Nature Catalysis on September 21.
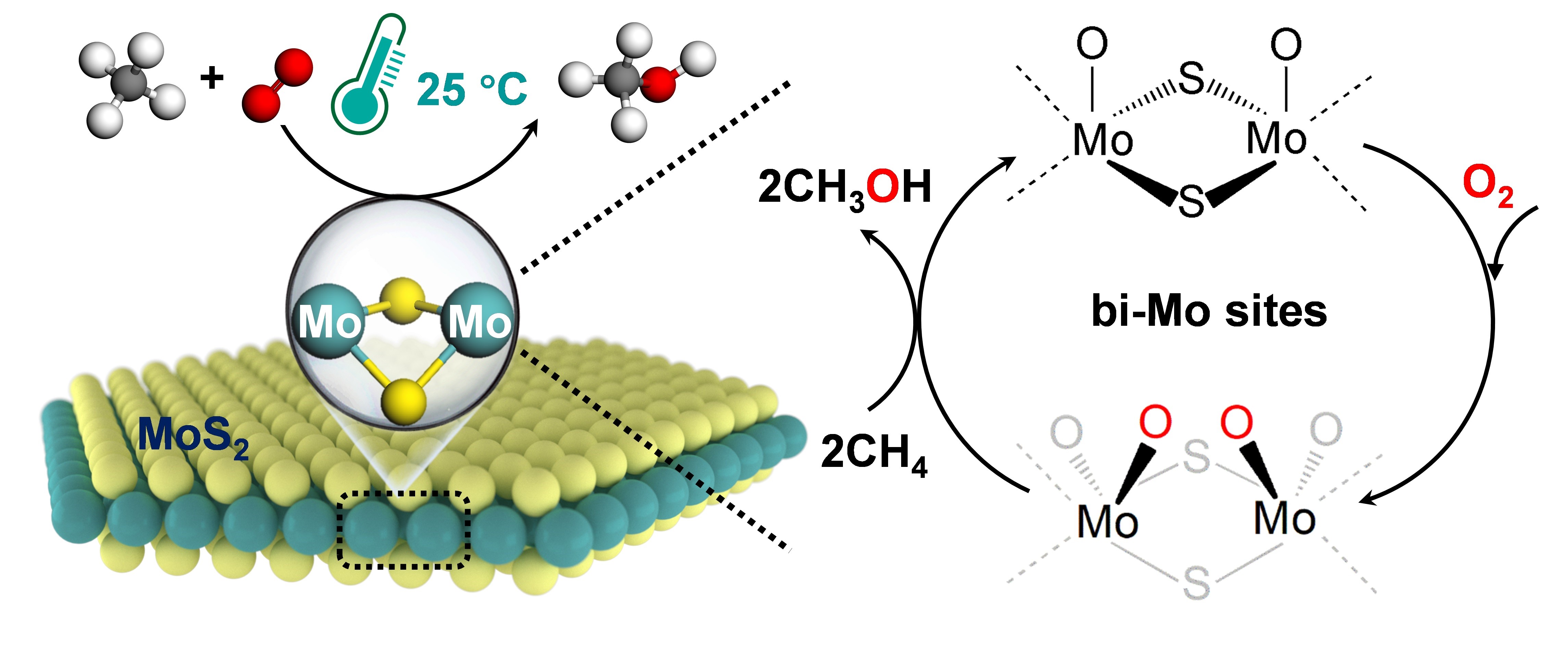
Room-temperature CH4 conversion by O2 over bi-Mo sites confined in MoS2 edge. (Image by MAO Jun and LIU Huan)
Catalytic conversion of methane to high value-added chemicals is an international tough problem due to the low polarization rate and high C-H bond energy (439 kJ mol-1) of methane, which is regarded as the "Holy Grail" in the field of chemistry. Typical catalytic conversion of CH4 usually operates at high temperatures (greater than 600 oC), or in the aid of strong oxidants (such as fuming sulfuric acid) or external fields (such as plasma). Nevertheless, such harsh reaction easily leads to excessive conversion of the target product (such as overoxidation to CO2).
Direct conversion of CH4 and O2 at low temperatures or even at room temperature is an appealing strategy for CH4 conversion, however, which is extremely challenging due to the great difficulty on the formation of active oxygen species continuously under mild conditions for C-H activation.
In this study, a remarkable CH4 conversion of up to 4.2% with a high selectivity of over 99% for the C1 oxygenates is achieved for CH4 conversion by O2 at room temperature. In situ characterizations combined with theoretical calculations demonstrated that the unique binuclear molybdenum (bi-Mo) site of sulfur vacancies at the MoS2 edge is able to directly dissociate O2 to form O=Mo=O* active species at 25 oC, which can activate the C-H bond of CH4 and thereby driving the catalytic conversion of CH4 to C1 oxygenates via CH3O* intermediates at room temperature.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China and the Strategic Priority Research Program of CAS. (Text by MAO Jun and CUI Xiaoju)
Link:
https://www.nature.com/articles/s41929-023-01030-2
http://www.dicp.ac.cn/xwdt/ttxw/202309/t20230922_6885298.html