Decoupled electrolysis of water is a promising strategy for peak load regulation of electricity. It could store the surplus electricity in the valley time and produce hydrogen with low energy consumption in the peak time. This strategy is useful to relieve the imbalance between the generation and consumption of electricity, which is significant to realize the efficient utilization of electric energy.
The key of developing this technology is to construct decoupled devices containing efficient catalysts and corresponding stable redox mediators, which still remains a considerable challenge.
Recently, Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) designed an efficient decoupled water device by using a kind of high-performance chainmail catalyst as the electrode. It realizes the peak shaving and valley filling by converting the surplus electricity to hydrogen energy.
Their study was published in The Innovation on July. 14.
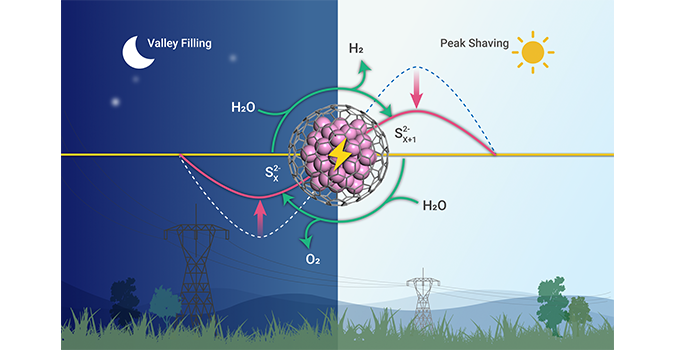
Efficient decoupled water electrolysis device realized peak shaving and valley filling of electricity.
The concept of “chainmail catalysis” is firstly proposed by the team of Deng Dehui in the world. And subsequently systematic researches have been carried out focusing on the structure design of catalysts and the activity regulation of reactions (Angew. Chem. Int. Ed. 2013, 52, 371, Angew. Chem. Int. Ed. 2014, 53, 7023, Energy Environ. Sci. 2014, 7, 1919, Angew. Chem. Int. Ed. 2015, 54, 2100, Nature Nanotech. 2016, 11, 218, Energy Environ. Sci. 2016, 9, 123, Adv. Mater. 2017, 29, 1606967, Adv. Mater. 2019, 31, 1901996, Energy Environ. Sci. 2020, 13, 119, Adv. Mater. 2020, 32, 1908126).
Based on that, researches from Deng’s group developed a decoupled water device by using the graphene-encapsulated CoNi chainmail catalyst as the electrode and polysulfides as mediators. This device produced hydrogen with a low potential of 0.82 V at 100 mA/cm2. Compared with the direct electrolysis of water, the potential of hydrogen production is reduced by 1.24 V, saving 60.2% energy in the peak time. With the efficient catalysis of chainmail catalyst, this device runs stably for 500 h at the current density of 500 mA cm-2, which reaches high capacity of 2.5*105 mAh cm-2 for producing hydrogen.
The reaction active sites were studied by DFT calculations cooperating with Prof. Guan Jing from Qingdao University of Technology. The superiority of chainmail catalyst originates from the modulation of the electronic structure of graphene surface by metal core and nitrogen dopant, as revealed by the combination of theory and experiment results.
“This work provides a new route to the rational use of electricity in a separate period, which is helpful to construct smart power grids in the practical applications” said Prof. DENG.
These works were supported by National Natural Science Foundation of China, Key Research Program of Frontier Sciences of the Chinese Academy of Sciences and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM).
Link: http://www.dicp.ac.cn/xwdt/kyjz/202108/t20210805_6151981.html