Recently, Prof. Dehui Deng from Group 05T6 in State Key Laboratory of Catalysis (SKLC) of Dalian Institute of Chemical Physics (DICP), reported a MoS2 nanofoam catalyst with co-confined Se in the surface and Co in the inner layer of the tri-atomic-layer structure of MoS2, which exhibits high catalytic activity and durability for acidic hydrogen evolution reaction (HER) at a large current density of 1000 mA cm-2. This study opens up a new prospect of tailoring the catalytic performance of MoS2 toward large-scale HER applications through confining multi-elements in different atomic layers.
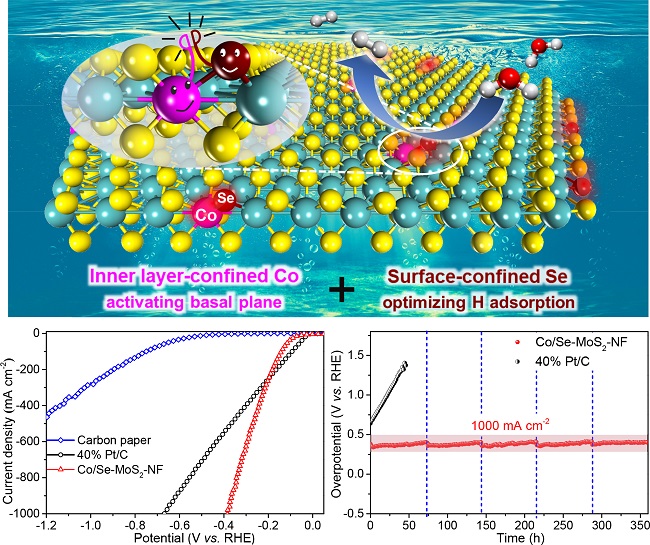
Two-dimensional (2D) MoS2, an earth-abundant material with unique geometric and electronic properties, has shown attractive catalytic activity for the HER and is considered as a potential alternative to the precious platinum-based catalysts in acidic medium. However, only the edge S sites of pure MoS2 are catalytically active for the HER and the vast amount of S sites in the basal plane are quite inert and not sufficiently utilized. Besides, the low stability of edges also limits the HER performance of the MoS2. Thus, selectively activating the inert basal plane combined with enriching and stabilizing the edges can maximally increase active sites of MoS2 for the HER, but is highly challenging owing to the difficulty in balancing the activity and stability.
Prof. Deng’s research group reported for the first time the strategy of confining transition metal atoms into the MoS2 lattice to trigger HER activity over the S atoms in the inert basal plane (Energy Environ. Sci., 2015, 8, 1594). Their latest study shows that co-confining Se in the surface and Co in the inner layer of the MoS2 can realize simultaneously activation of the basal plane, optimization of the hydrogen adsorption activity, and stabilization of the structure. Such a catalyst exhibits an ultra-high HER activity in acidic medium. At a large current density of 1000 mA cm-2, a much lower overpotential of 382 mV than that of 671 mV over commercial Pt/C catalyst is achieved and stably maintained for 360 hours without decay. The activity surpasses those of all previously reported heteroatom-doped MoS2. Density functional theory calculations demonstrate that inner layer-confined Co atoms stimulate neighbouring S atoms while surface-confined Se atoms stabilize the structure, which cooperatively enable the massive generation of both in-plane and edge active sites with optimized hydrogen adsorption activity.
Prof. Deng’s research group focus on modulating the surfaces and interfaces of 2D materials for energy catalysis and has made a series of research progress in developing 2D material-based catalysts for water electrolysis (Adv. Mater., 2020, 32, 1908126; Angew. Chem. Int. Ed., 2020, 59, 10502; Nano Energy, 2020, 72, 104700; Nano Energy, 2019, 61, 611; Chem. Rev., 2019, 119, 1806; Nano Energy, 2018, 52, 494; Nat. Commun., 2017, 8, 14430; Nat. Nanotechnol., 2016, 11, 218; Energy Environ. Sci., 2016, 9, 123; Angew. Chem. Int. Ed., 2015, 54, 2100; Energy Environ. Sci., 2015, 8, 1594; Energy Environ. Sci., 2014, 7, 1919; over ten patents applied). Recently, they have successfully developed a portable hydrogen-oxygen generator with integrated non-noble metal electrode catalysts, which has been mass produced and has obtained the China GB, European Union RoHS and CE, and American FDA certificates, and can be put into markets in the Asia, Europe, and America. The findings in this study provide an important guiding for further optimization and upgrade of the electrode catalyst of the portable hydrogen-oxygen generator.
This work has been published as a research article in Nature Communications. This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, the DNL Cooperation Fund, CAS, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM). (Text by Zhilong Zheng, Liang Yu and Hehua Gao)
Link:http://www.dicp.ac.cn/xwdt/ttxw/202007/t20200713_5623797.html