Recently, Prof. Dehui Deng and Assoc. Prof. Liang Yu from Group 05T6 in State Key Laboratory of Catalysis (SKLC) of Dalian Institute of Chemical Physics (DICP), reported a distance synergy of MoS2-confined single-atom rhodium realizing an ultra-high hydrogen evolution reaction (HER) activity at the in-plane S sites adjacent to the rhodium. This work offers new insights into the effect of confining single heteroatoms in tuning the catalytic activity of MoS2, and provides a new strategy of designing high-performance HER catalyst.
Two-dimensional (2D) molybdenum disulfide (MoS2) has attracted wide attention as a potential HER catalyst because of the unique activity for hydrogen adsorption at the edge S sites. Vast numbers of S atoms in the wide-open basal plane of MoS2, though chemically inert, also offer opportunities of exploiting active centers over the 2D surface, which is still a great challenge.
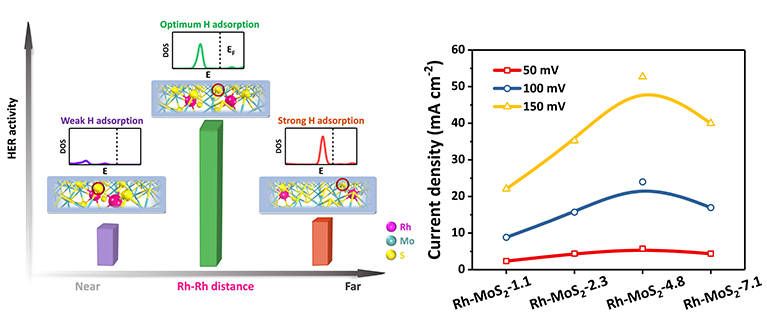
Prof. Dehui Deng's research group focus on designing advanced 2D materials and modulating their surfaces and interfaces to trigger efficient catalytic activities. They firstly reported the strategy of confining transition metal atoms into the MoS2 lattice to efficiently modulate the in-plane reactivity of the neighboring S atoms (Energy Environ. Sci., 2015, 8, 1594; Nat. Nanotechnol., 2016, 11, 218; Nat. Commun., 2017, 8, 14430; Nano Energy, 2019, 61, 611; Chem. Rev., 2019, 119, 1806). In this latest study, they reported a MoS2-confined single-atom Rh electrocatalyst realizing an ultra-high HER activity. The average inter-Rh distances in different samples were controlled by regulating the doping concentration of Rh atoms in the Rh-MoS2, leading to the highest HER activity at a proper inter-Rh distance. With a Rh weight percent of 4.8 %, an ultra-low overpotential of 67 mV versus the reversible hydrogen electrode is achieved at a current density of 10 mA cm-2, which surpasses the activity of mostly reported MoS2-based catalysts in acidic solution and is closer to that of the standard 40 % Pt/C catalyst. Density functional theory (DFT) calculations demonstrate a synergy between the confined Rh atoms which leads to a volcanic type relation between the limiting potential of the HER and the inter-Rh distance in the MoS2 lattice. A proper inter-Rh distance can induce an optimum activity on the neighboring S atoms for H adsorption.
This work has been published as a communication in Angew. Chem. Int. Ed. This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, the DNL Cooperation Fund of CAS, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM). (Text by Xiangyu Meng and Hehua Gao)
Link:http://www.dicp.cas.cn/xwdt/ttxw/202005/t20200506_5568511.html