Research groups led by Prof. XIAO Jianping and Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences recently proposed a new routine for electrochemical ammonia synthesis from nitric oxide (NO), which is supposed from exhausted gases of thermal power stations, factories, or vehicles. This work was published in the journal of Angew. Chem. Int. Ed.
NO is one of the major air pollutants, which usually comes from the combustion of fossil fuels in power plant and automobile. Therefore, NO removal is essential for the waste gas treatment in industry.
In the meanwhile, ammonia synthesis is another important chemical process in industry. Ammonia is a basic chemical substance in chemical production, which can be used to produce fertilizer, nitric acid and explosives. The traditional method of ammonia synthesis mainly depends on Haber-Bosch process, while this process can only happen under high temperature and pressure, which needs a lot of energy input.
The electrchemical nitrogen reduction reaction can be carried out at ambient conditions, but it suffers from the low activity and selectivity due to the chemical inertness of N2 molecule. In this work, XIAO ea al. proposed to synthesize ammonia from exhausted NO via electrocatalysis.
NO electroreduction to ammonia. (Image by LONG Jun, LI Jiayi)
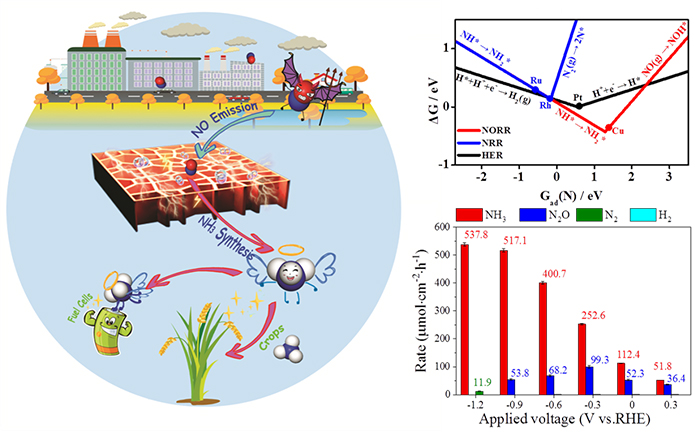
Based on the density functional theory calculations, ten reaction pathways were considered to study the NO reduction (NORR) mechanism. It’s found that NO reduction was more feasible than the N2 reduction and hydrogen evolution reaction in thermodynamics, and Cu was screened as the best transition metal catalyst via a descriptor based approach.
Kinetic calculations suggested NH3 was the most preferred production for NORR on Cu(111) surface. In addition, experiments revealed Cu Foil was more selective than Pt Foil, which was consistent with our theoretical calculations. On Cu Foam electrode, a record-high ammonia synthesis rate of 517.1 μmol/(cm2·h) and FE of 93.5% were achieved at -0.9 V vs. RHE, with a stable electrocatalytic performances in 100 hours run.
At last, a microkinetic simulation was performed to estimate the turnover frequency (TOF) at different potentials, which exhibited a nice linear correlation with the experimental ammonia yield rate, confirming our reaction pathways and mechanisms above.
This work was supported by the National Natural Science Foundation of China, the National Key R&D Program of China and LiaoNing Revitalization Talents program etc. (Text by LONG Jun, LI Jiayi)
Link: http://www.dicp.cas.cn/xwdt/kyjz/202003/t20200330_5521899.html