Recently, Prof. Dehui Deng and Prof. Xinhe Bao's group from SKLC, DICP, reviewed the latest progress in methane conversion under mild conditions in thermocatalysis, electrocatalysis, and photocatalysis systems. This work has recently been published in Chem (Chem, 10.1016/j.chempr.2019.05.008).
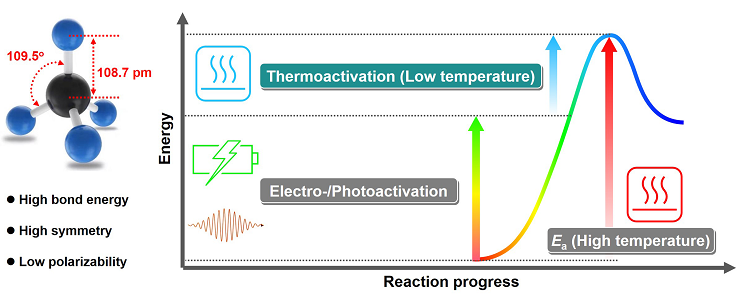
Fig 1 Molecular Structure of Methane (Left) and Energy Diagram for Methane Activation in Electro-/Photoactivation Involved Reaction Systems (Right).
Methane is an abundant natural resource and the basis of natural gas, shale gas, combustible ice, etc. It is not only a clean energy widely used today, but also a C1 building block for producing many industrial chemicals, which has aroused intense interest from both academic and industrial communities. However, the stable nonpolar structure and extremely high dissociation energy of C-H bond make the activation of methane molecule very difficult, which is regarded as the “holy grail” in catalysis. In the conventional process that has been industrially implemented, methane conversion in large scale is through the steam-methane reforming to produce syngas (a mixture of CO and H2 as the feedstock of FT synthesis) and conversion of syngas in further steps to obtain various chemicals. In general, the steam-methane reforming in the first step requires very high temperature (700 oC-1100 oC) and the syngas conversion in the subsequent step also needs high temperature and high pressure, which increase both economic and environment costs. Therefore, developing direct low-temperature methane conversion technologies for the production of high value-added chemicals is an urgent but challenging subject.
In this article, typical catalysts employed in various reaction systems (thermocatalysis, electrocatalysis, and photocatalysis) for methane conversion under mild conditions were summarized. Especially, the heterogeneous catalysts with noteworthy C-H activation performance were highlighted. The topics of catalyst design, theoretical simulations, choice of reaction condition, and method of reaction product analysis were also discussed. As important perspectives presented in this review, it is still attractive to develop high-performance catalysts that can use O2 as oxidant in methane conversion. In addition, design of confined single site catalysts and multi-component catalysts, especially the materials that can couple multiple driving forces from thermal, electric, and solar energy with excellent C-H activation ability should be crucial to the development of more efficient low-temperature pathways for methane conversion.
Prof. Deng and Prof. Bao's group has been focusing on the development of 2D material-based catalysts and their applications in the catalytic conversion of energy-related molecules (Nature Nanotechnology, 2016, 11, 218; Chemical Reviews, 2019, 119, 1806). As early as 2015, this group reported the capability of graphene-confined single iron sites for the catalytic oxidation of complicated hydrocarbons at room temperature (Science Advances, 2015, 1, e1500462). Notable recent progress by this group includes the finding that graphene-confined single iron atoms could even catalyze methane conversion at room temperature (Chem, 2018, 4, 1902). These results demonstrate bright prospects for 2D material -based catalysts in the application of C-H activation process.
These works are supported by Ministry of Science and Technology of China, Major Project of National Natural Science Foundation of China, Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, and the DNL Cooperation Fund, etc. This work is dedicated to the 70th anniversary of Dalian Institute of Chemical Physics, CAS. (by Xianguang Meng and Hehua Gao)
Link: http://www.dicp.cas.cn/xwdt/kyjz/201906/t20190603_5312121.html