Recently, Prof. Dehui Deng’s group from 05T6, SKLC, DICP, first reported a room-temperature electrochemical water–gas shift process for direct production of high purity hydrogen (over 99.99%). This work has recently been published as a research article in Nature Communications (Nat. Commun. 2019, 10, 86).
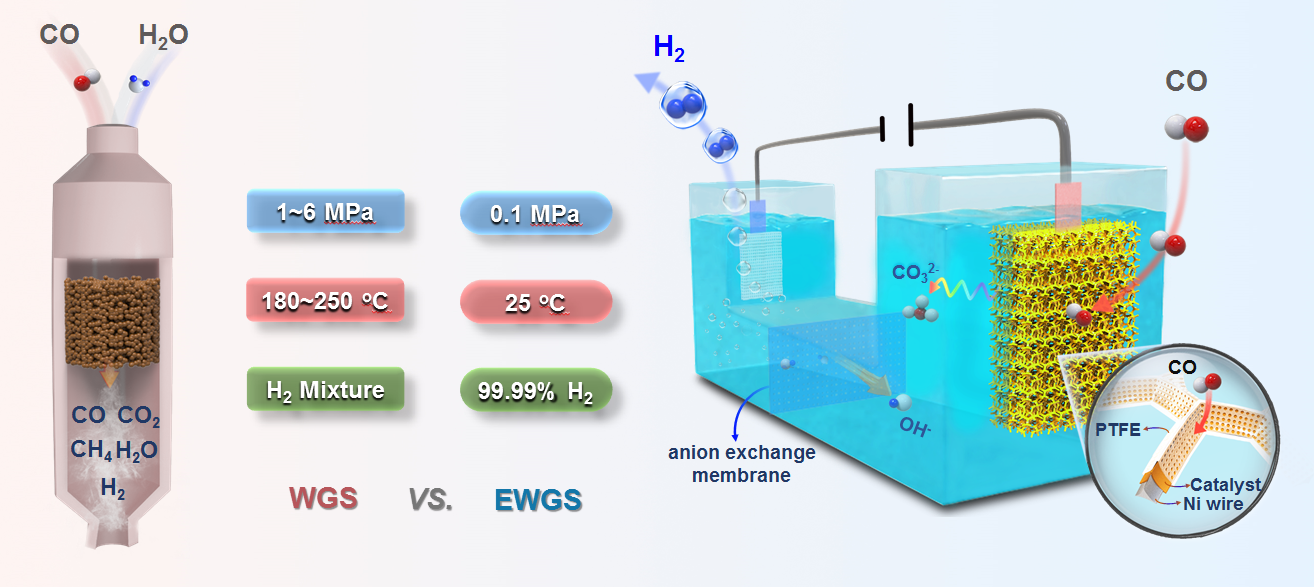
Fig 1 Room-temperature electrochemical water-gas shift reaction
for high purity hydrogen production
Hydrogen is the most potential for clean energy in the 21 century. The water-gas shift (WGS) reaction is a key step in carbon-based energy processes for large-scale hydrogen production The process is typically operated at 1.0-6.0 MPa under high temperatures (180 oC-250 oC) to overcome the sluggish reaction kinetics. Apart from the harsh conditions, H2 produced by the WGS reaction contains CO residuals (around 1%-10%) and significant quantities of CO2 and CH4, etc., which needs extra processes of separation and purification so as not to affect the downstream applications. Therefore, the direct production of high purity hydrogen under mild conditions by a more economical and eco-friendly strategy is the urgent requirements for development of hydrogen energy but also a challenging task.
Electrochemically decoupling the WGS redox reaction to separated cathodic reduction reaction and anodic oxidation reaction in an electrolytic cell and using electric-potential as the driving force of the process is a promising approach of circumventing the aforementioned harsh conditions. Herein, we report for the first time a completely new process of directly producing high purity hydrogen (over 99.99%) with low energy cost and high energy conversion efficiency, i.e. the room-temperature electrochemical water-gas shift (EWGS) process. In the EWGS process, CO is oxidized on the anode, where the product CO2 further reacts with hydroxide ion forming CO32- and thereby is transformed into a potassium carbonate avoiding the pollution caused by CO2 emission. Meanwhile, high purity H2 is produced from H2O reduction on the cathode. Anion exchange membrane was employed for separating the cathode and anode, maintaining the balance of ion concentration of electrolyte, and avoiding the separation process in WGS in principle. Through rational design of anode structure to facilitate CO diffusion and PtCu catalyst to optimize CO adsorption, the electrolysis process can be effectively driven at room temperature and atmospheric pressure with a faradaic efficiency of approximately 100%. Comparing with the 1.23 volts for the electrolysis of water, the anodic onset potential is lowered to almost 0 volts at room temperature and atmospheric pressure. The optimized PtCu catalyst achieves a current density of 70.0 mA cm-2 at 0.6 volts which is over 12 times that of commercial Pt/C (40 wt.%) catalyst, and remains stable for even more than 475 hours. Besides, cooperating with Prof. Hai-Yan Su, combining DFT, we found that the CO adsorption on Pt site of Pt3Cu is weakened which is the reason for high activity of EWGS. This study achieves an original innovation for a new process of hydrogen production via multidisciplinary crossing. Considering the abundant CO feedstock, and the advantages of low energy input and high energy conversion efficiency in contrast to either WGS or water electrolysis, the EWGS process provides a promising route to produce high purity hydrogen in the future.
These works are supported by Ministry of Science and Technology of China, National Natural Science Foundation of China, Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, the DNL Cooperation Fund and National Postdoctoral Program for Innovative Talents, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM). (by Ruixue Chen and Hehua Gao)
Link: http://www.dicp.ac.cn/xwdt/kyjz/201901/t20190114_5228524.html