The research about the “chainmail” catalysis by Prof. Dehui Deng and Prof. Xinhe Bao etc. from SKLC, DICP have attractedgreat interests byinternational counterparts. Recently they have been invited to publish a review paper entitled “Robust Catalysis on 2D Materials Encapsulating Metals: Concept, Application, and Perspective (Adv. Mater. DOI: 10.1002/adma.201606967)” in Advanced Materials.
Search for low-cost and earth-abundant non-precious-metal catalysts to replace the rare precious metals and realize the high-efficient conversion of significant energy and chemical engineering processis a focusedresearch in today’s catalysis fields. While under some harsh reaction conditions, the instability of non-precious metals is an urgent problem to be overcome. Recently, on the basis of previous studies towards nano-catalysis, Prof. Dehui Deng and Prof. Xinhe Bao etc. from SKLC, DICP have innovated the prepared strategy of nano-catalysts and successfullysynthesized graphene shells encapsulating 3 dtransition metal nanoparticles. Experiments and DFT calculations indicate that encapsulating the active metal nanoparticles into the graphene shells canefficientlyseparate the metal nanoparticles from the external harsh conditions (e.g. strong acid and base, and strong corrosion etc.), and therefore hinder the catalyst’s deactivation during the catalytic reaction process.Meanwhile, the active electron of encapsulated metal nanoparticles willpenetrate through the graphene layer to the outermost surface, thereby leading to a high catalytic activity on the graphene surface. The correlated concept has been widely accepted and described vividly as “chainmail for catalyst”.(Angew. Chem. Int. Ed.;Nat. Nanotechnol.)
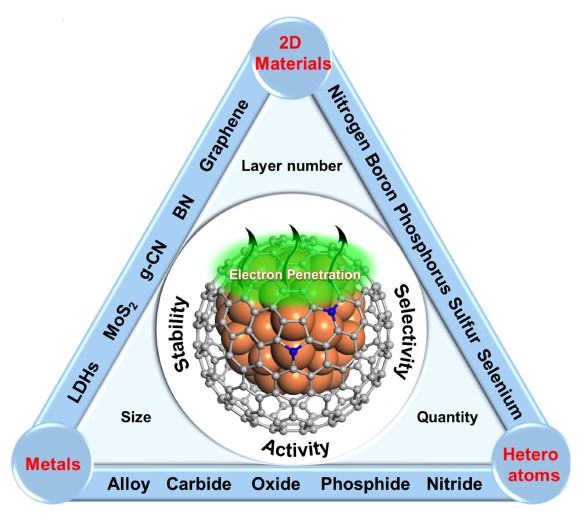
In recent years, rapid progress has been made towards this concept in electrocatalysis, photocatalysis and heterogeneous catalysis etc.The Prof. Bao and Deng’s group, who initially developed this concept, has also madeseveral important progress in the conversion and utilization of energy-related small molecules. Especially, in the oxygen reduction of fuel cells (J. Mater. Chem. A), electrocatalytic hydrogen evolution (Energy Environ. Sci.; Angew. Chem. Int. Ed.), electrocatalytic oxygen evolution (Energy Environ. Sci.), dye-sensitized solar cells (Angew. Chem. Int. Ed.), Li-oxygen battery (Nano Energy), syngas conversion (Chin. J. Catal.). In addition, through the photoemission electron microscopy (PEEM) and soft X-ray imaging technology, electronic structure modulation of carbon layer surface by the active metals has been directly observed. Then, combined with the theoretical calculations, the nature of interaction between metal and carbon has been demonstrated (Chem. Sci.), promoting the understanding about the “chainmail” catalyst.
These works are supported by the Ministry of Science and Technology of China, National Natural Science Foundation of China, Strategic Priority Research Program of the Chinese Academy of Sciences, Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, and Collaborative Innovation Center of Chemistry for Energy Materials.(2011 iChEM)(Text and Image by Jiao Deng)
Link:http://www.dicp.cas.cn/xwdt/kyjz/201811/t20181119_5187779.html