Directly converting methane (CH4) to high value-added multi-carbon (C2+) oxygenates, such as acetic acid (CH3COOH), under mild conditions presents a promising pathway for upgrading natural gas to transportable liquid chemicals.
The direct coupling of CH4, carbon monoxide (CO) and oxygen (O2) to produce CH3COOH represents a significant pathway for converting natural gas into high-value-added chemicals. Although this reaction system exhibits notable thermodynamic advantages at low temperatures, achieving efficient and selective conversion of CH4-to-CH3COOH through C-C coupling remains great challenging due to key scientific obstacles including high activation barriers of C-H bonds in CH4, difficult dissociation of O2, and poor controllability of C-C coupling.
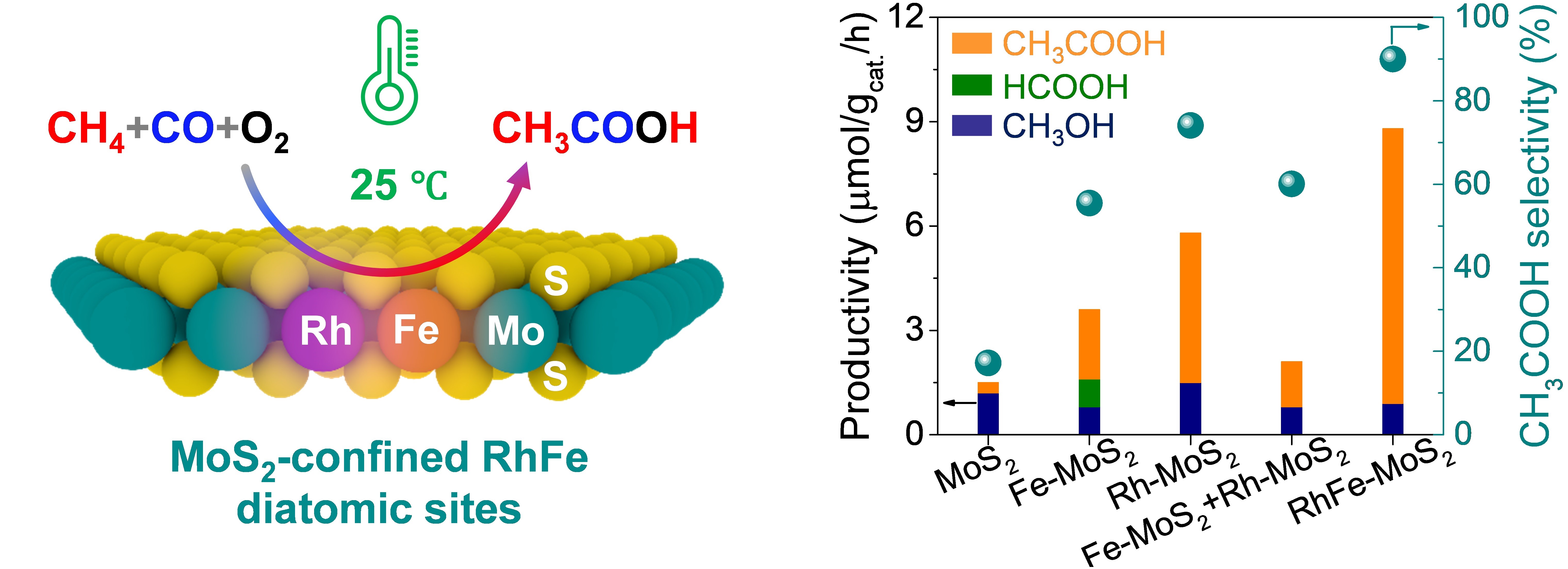
Schematic illustration of the CH4 carbonylation with CO and O2 to CH3COOH over the RhFe-MoS2 and the comparison of catalytic activity for different catalysts (Image by MAO Jun and LIU Huan)
In a study published in Journal of the American Chemical Society, a research group led by Prof. DENG Dehui, Assoc. Prof. CUI Xiaoju, and Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) have successfully achieved highly efficient carbonylation of CH4 with CO and O2 to CH3COOH over MoS2-confined Rh-Fe dual-site at low temperatures and even at room temperature. This catalyst delivers an unprecedented CH3COOH selectivity of 90.3% and a productivity of 26.2 μmol gcat.–1 h–1 at 25 °C, significantly outperforming the previously-reported catalytic systems.
Moreover, the researchers revealed that the confined Fe sites in MoS2 enable the activation of O2 to generate highly reactive Fe=O center for CH4 dissociation to CH3 species at room temperature, which then readily couple with adsorbed CO on adjacent Rh sites to form the key CH3CO intermediate for CH3COOH production. The unique structure of Rh–Fe sites offer synergistic catalytic properties that effectively balance C–H activation and C–C coupling, successfully addressing the trade-off between activity and selectivity in the carbonylation of CH4 to CH3COOH under mild conditions.
"Our study opens new avenues for the design of efficient catalysts for the oxidative carbonylation of CH4 to CH3COOH." said Prof. DENG.
Link:
https://www.dicp.ac.cn/xwdt/kyjz/202505/t20250512_7650226.html
https://pubs.acs.org/doi/10.1021/jacs.5c01515